Pioneering a New Era in Immunotherapy
EnnoDC I About
EnnoDC is a clinical-stage biotech company pioneering the development of first-in-class dendritic cell-targeting CD40 and antigen-specific immunotherapies for cancer and infectious diseases.

We have a growing pipeline of novel immunotherapies, several of which are in clinical development. Target cancers include HPV16+ oropharyngeal and prostate cancer. Infectious disease targets include COVID and HIV.
Our groundbreaking and versatile technology platform harnesses the power of immunotherapy and vaccines by stimulating dendritic cells (DCs) to deliver a more targeted and effective immune response compared to current treatments.
EnnoDC I Mission
Our mission is to develop the next generation of immunotherapies using the latest scientific insights to develop antibodies for patients affected by cancer and infectious diseases.

EnnoDC I Team
We are led by a seasoned management team with internationally recognised expertise in medical, industry and business.
I Use the filters below to learn more about the EnnoDC team
Management Team
Supervisory Board
Scientific Advisory Committees
EnnoDC I Partners
EnnoDC, the trading name of LinKinVax, is a spin-off of the academic laboratory, the Vaccine Research Institute (VRI) an institute of INSERM (the French National Institute of Health and Medical Research).

The EnnoDC team includes European and US Board members and scientific advisors, and the company has a long-standing collaboration with the Baylor Institute for Immunology Research (Dallas, TX).
EnnoDC is pursuing and developing new collaborations with several internationally regarded cancer institutions including Moffitt Cancer Centre in the US and Gustave Roussy Cancer Campus in France.

In addition, EnnoDC is one of the innovative projects selected and supported by the Paris Saclay Cancer Cluster, whose mission is to accelerate the development of innovation in oncology.
EnnoDC I Science
Our groundbreaking and versatile antibody technology harnesses the power of immunotherapy and vaccines by stimulating dendritic cells (DCs) to deliver a more targeted and effective immune response compared to current treatments.

Anti-CD40
I Immunotherapy activation
EnnoDC uses a novel, humanized and versatile anti-CD40 antibody. This antibody acts as a signal booster, stimulating DCs to educate and stimulate the immune system. Dendritic cells (DCs) are the most potent antigen-presenting cells in the immune system and activated DCs are crucial for the development of immune memory to identify and eliminate threats such as cancer and viruses.
I Vaccine specificity
EnnoDC’s anti-CD40 antibody can be tailored to incorporate specific antigens from cancer cells or pathogens (viruses, bacteria). These antigens are delivered directly to the activated DCs, guiding the immune system to launch a targeted attack against the intended threat.
An immunotherapy combining mAbs and a Commutable Antigen targeting Dendritic Cells (DCs) through CD40.
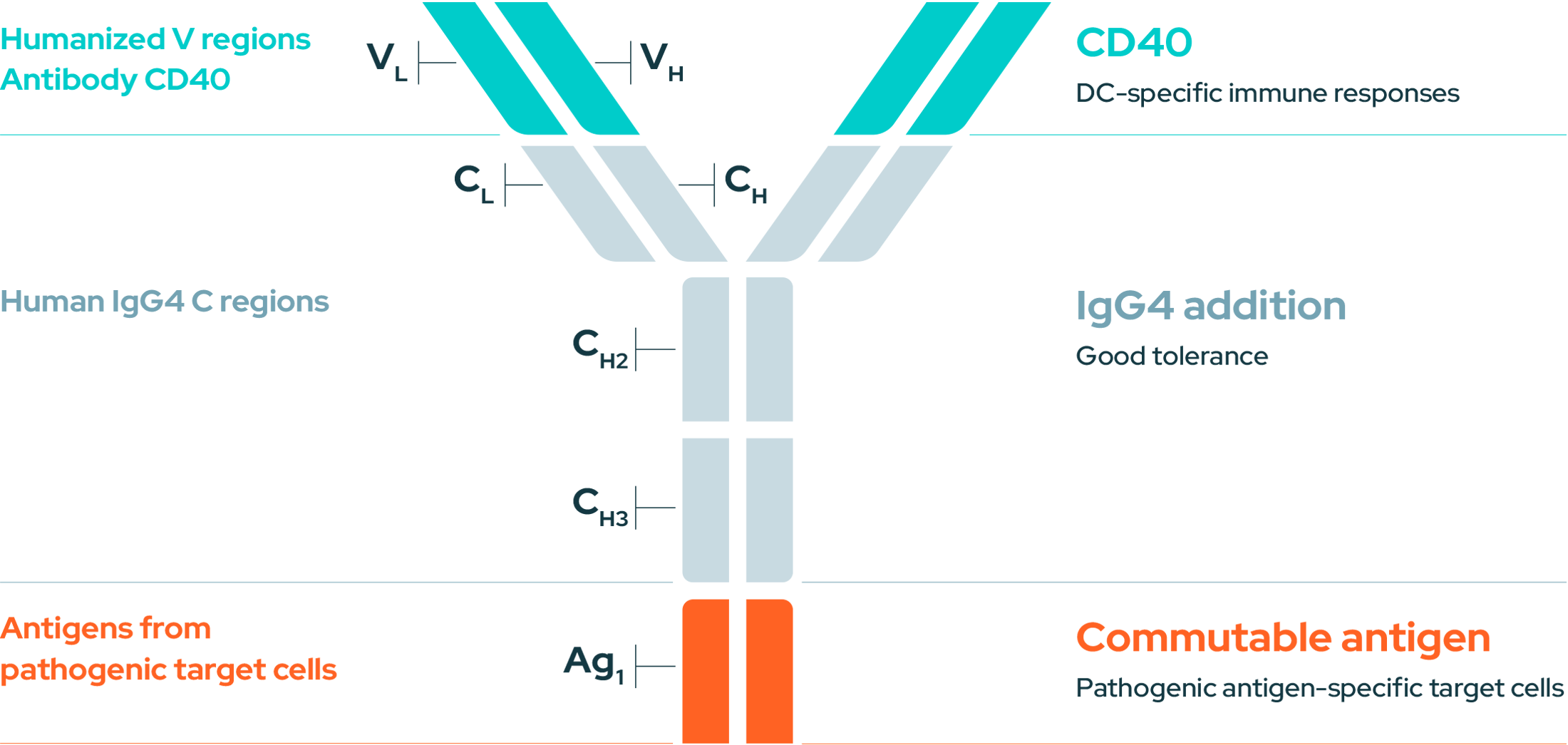
I Advantages of our approach
01. Dual action
EnnoDC offers a first-in-class antibody which combines the benefits of immunotherapy and vaccines. It activates the immune system while directing it towards a specific target.

02. Enhanced specificity
By delivering antigens directly to DCs, EnnoDC promotes a more focused immune response, minimizing potential side effects.

03. Promising future
EnnoDC holds immense potential for various applications, including battling cancer and fighting infectious diseases.

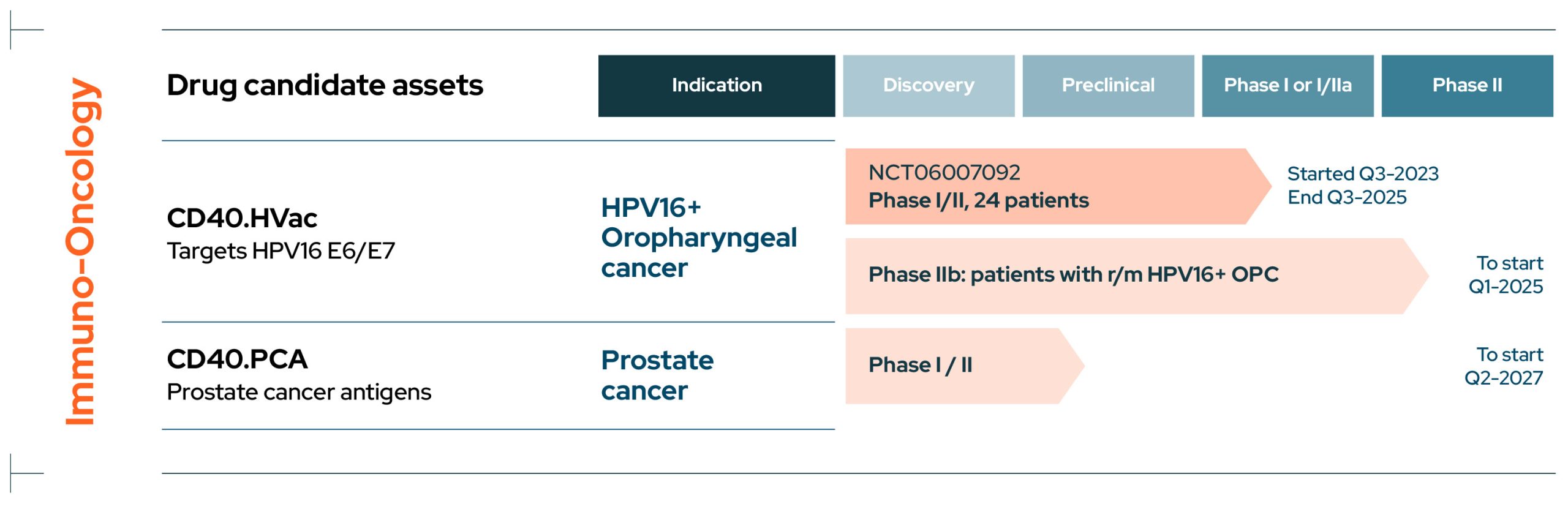
EnnoDC I Pipeline

Pipeline
I Development Pipeline
EnnoDC has a growing pipeline of novel immunotherapies for cancers such as HPV16+ oropharyngeal and prostate cancer, as well as infectious diseases including COVID and HIV.
Cancer
CD40.HVac
CD40.HVac is an immunotherapy targeting the human papilloma virus HPV16 E6/E7 antigen in patients with HPV16+ oropharyngeal carcinoma and is currently in an ongoing Phase I/II multicentric double-blind placebo-controlled dose escalation trial (NCT06007092).
The study is being conducted in collaboration with the Gustave Roussy Cancer Campus.
CD40.PCA
CD40.PCA is an immunotherapy targeting prostate cancer antigens and is currently in the preclinical development stage.
Infectious Diseases
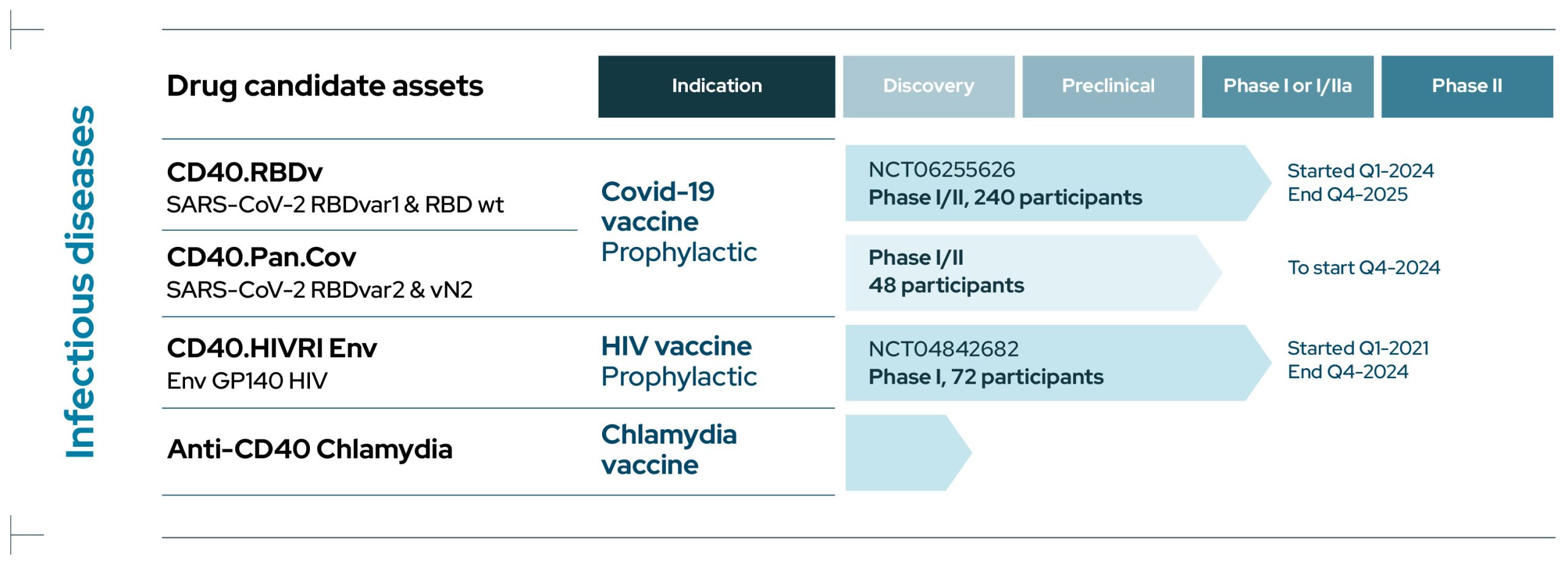
CD40.RBDv
CD40.RBDv is a prophylactic bivalent COVID-19 subunit protein vaccine containing both the ancestral SARS-CoV-2 sequence of RBD and the same portion of RBD harboring several mutations shared by several Variants of Concern (VOCs), including Omicron.
CD40.RBDv is currently evaluated in a Phase I/II clinical trial to test the safety and immunogenicity of the vaccine, adjuvanted or not, as a booster in volunteer (NCT06255626). This study is being conducted in collaboration with ANRS-MIE/Inserm.
CD40.Pan.Cov
CD40.Pan.Cov is a prophylactic COVID-19 vaccine aiming at boosting and extending pre-existing immune responses to antigens from various proteins of the SARS-CoV-2.
It is designed to target conserved regions from current SARS CoV-2 variants of concerns and Sarbecoviruses including RBD from spike harboring several mutations from VOCs and a highly conserved region from Nucleocapsid rich in T cell epitopes .
This vaccine is developed as a booster with a Phase I/II clinical trial due to start in Q4 2024 in collaboration with ANRS-MIE/Inserm.
CD40.HIVRI Env
CD40.HIVRI Env is a prophylactic vaccine targeting the Envelope of a clade C Human Immunodeficiency Virus (HIV).
In 2021-2024, a multicenter double-blind placebo-controlled Phase I/IIa dose-escalating trial was performed demonstrating safety and long-term immunogenicity of the vaccine in healthy volunteers in France and Switzerland (NCT04842682) in collaboration with ANRS-MIE/Inserm.
A second trial will start in 2025 in collaboration with the HVTN (HVTN318).
CD40.Chlamydia
We are conducting discovery research for a Chlamydia vaccine using EnnoDC CD40-vaccine technology.
Two candidate vaccines are under evaluation at the preclinical stage of development.
Filter
EnnoDC I Contact
If you wish to contribute, collaborate or learn more about EnnoDC, we would be delighted to hear from you.
Please contact us at christophe.hubert@ennodc.com